Table of Contents
ToggleLumbar Pedicle Screw Placement: How to Enhance Spine Stabilization
Traditional methods used for lumbar pedicle screw placement often face challenges such as high costs, intensive training, and lengthy procedures. However, Ruthless Spine’s innovative RJB system offers an alternate solution that simplifies the process while enhancing efficiency.
Understanding Lumbar Pedicle Screw Placement
What is Lumbar Pedicle Screw Placement?
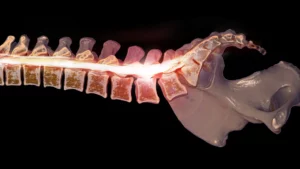
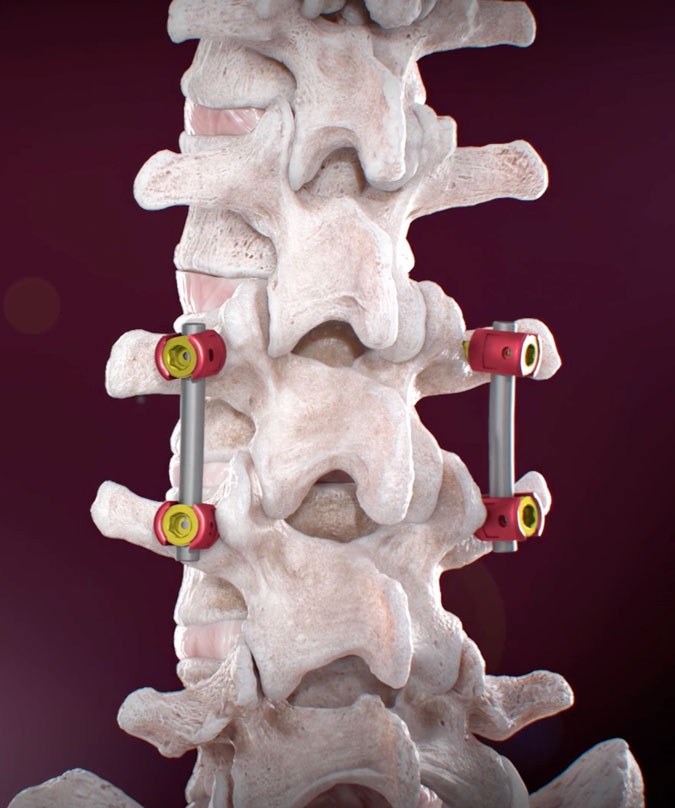
Lumbar pedicle screw placement is a surgical technique used to stabilize the spine by inserting screws into the pedicles of the vertebrae. This procedure is essential in treating various spinal conditions, including fractures, degenerative disc disease, and scoliosis.
Challenges in Traditional Methods of Lumbar Pedicle Screw Placement
Traditional methods, such as freehand techniques and fluoroscopy-guided systems, often come with significant drawbacks. These include the need for extensive preoperative planning, high radiation exposure, and the potential for screw misplacement, leading to complications and revision surgeries.
Ruthless Spine’s RJB System: A Revolutionary Solution
Overview of the RJB System
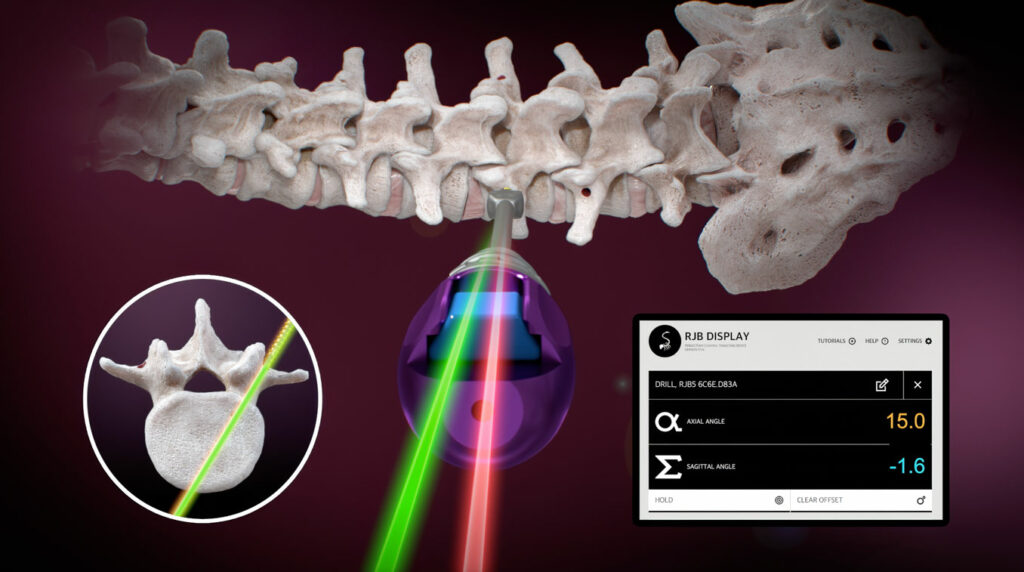
Ruthless Spine’s RJB system is an intraoperative surgical angle measurement tool designed for lumbosacral pedicle screw procedures. This FDA-cleared device offers a simplified yet highly effective approach to spinal surgery, leveraging modern technology.
Key Features and Benefits
Simplified Setup and Use
The RJB system is compatible with off-the-shelf Android or iOS tablets, eliminating the need for bulky and expensive machinery. Surgeons can set up the system in under five seconds, significantly reducing operating room time.
Continuous Connectivity
The Bluetooth-connected module ensures that the device maintains a reliable connection throughout the procedure, even if the line of sight is blocked.
Real-Time Visualization
The RJB provides real-time visualization of the axial and sagittal trajectory of the instrument shaft on the tablet screen. This feature allows for precise adjustments during surgery.

FDA Clearance and Compliance
The RJB system has received FDA clearance under De Novo classification, affirming its safety and effectiveness for use in lumbar pedicle screw procedures. This regulatory milestone underscores Ruthless Spine’s commitment to providing innovative and compliant solutions for spine surgery.
For the full instructions on using the RJB, refer to our ‘RJB User Guide’.
Ruthless Spine’s Mission
Ruthless Spine’s mission is to revolutionize spine surgery by simplifying and enhancing surgical navigation. They aim to bring “zen” back to spine surgery by addressing the challenges faced by spinal surgeons, such as the need for significant investments in capital, time, and manpower to achieve accurate outcomes. Ruthless Spine focuses on providing innovative, accurate, and efficient solutions that are easy to use and do not require bulky, complex machinery.
References
- Ruthless Spine RJB Information. (2023). Retrieved from Ruthless Spine
- FDA Medical Devices Databases. (2023). Device Classification Under Section 513(f)(2)(De Novo). Retrieved from FDA
- Ruthless Spine Company Information. (2023). Retrieved from Ruthless Spine
- Lee, H. M., Suk, K. S., Kim, J. T., & Moon, S. H. (2014). The long-term effect of instrumentation removal after posterolateral lumbar fusion. Asian Spine Journal, 8(2), 212-220. https://doi.org/10.4184/asj.2014.8.2.212