The RJB™ | IFU
RJB-IFU1-01 Rev B – Released 10/24/2023
US Patent No. 11,484,381; Additional US and foreign patents pending
IMPORTANT INFORMATION FOR PHYSICIANS, SURGEONS, AND STAFF
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
“This device complies with part 15 of the FCC rules. Operation is subject to the following two conditions.
1. This device may not cause harmful interference.
2. This device must accept any interference received, including interference that may cause undesired operation.”
Table of Contents
Select any chapter directory below to navigate directly to its section.
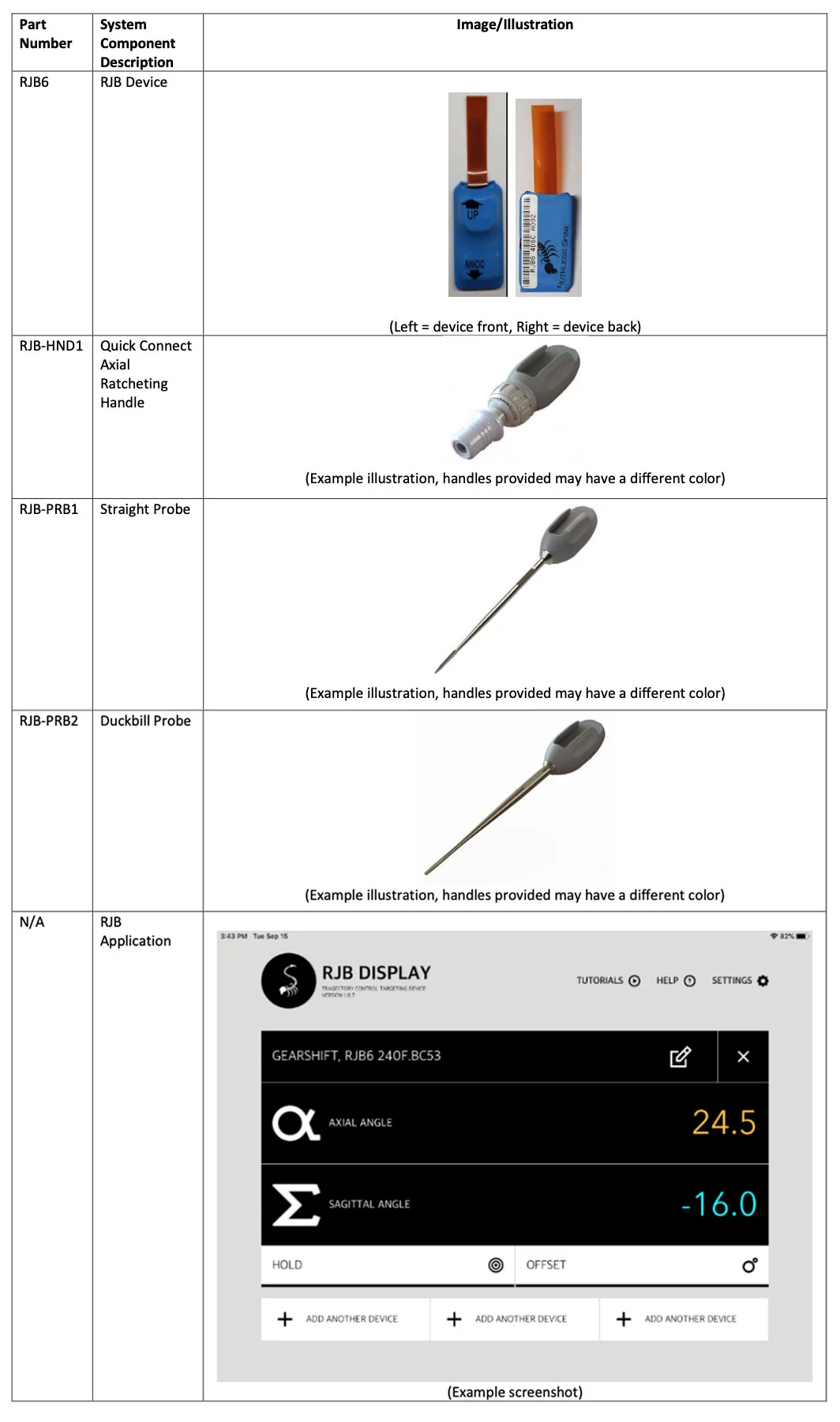
Device Description
The Ruthless Spine RJB device is an intraoperative surgical angle measurement guide that attaches to surgical instruments to measure the angle of the instrument relative to a vertical plumb line in line with gravity. The device can measure the axial and sagittal angles relative to gravity. The RJB system only provides measurements for angles in two planes relative to the vertical gravitational plumb line. As such, the RJB device does not provide surgical assistance, guidance, or navigation against patient anatomy. The RJB device is not intended to replace a surgeon’s clinical judgement and has not undergone clinical evaluation. No clinical benefit has been demonstrated or is claimed.
The RJB device is provided sterile for single use and utilizes Bluetooth Low Energy (BLE) to connect to a tablet computer and display the angle measurements via the RJB Application (App). A set of handles and instruments compatible with the RJB are provided with the device for use in lumbosacral pedicle screw placement. The following components are part of the RJB system:
- RJB Device
- Quick Connect Axial Ratcheting Handle
- Straight Probe
- Duckbill Probe
- RJB Application
Note, a Tablet Computer is required to operate the device. The Tablet is not provided to the end user.
See Appendix A for a complete listing of system components, part numbers, and accompanying images/illustrations.
Device Characteristics
Model: RJB6
Precision of Displayed Values: User choice of 0.5 or 1° in app
Procedural Measurement Accuracy*: ±3°
*Measurements made relative to a vertical plumb line in line with gravity. This measurement accuracy was determined through simulated use testing of the device in a lumbar pedicle screw placement procedure.
Operating Condition: -18 – 55°C, 15 – 90% relative humidity, 70 – 106 kPa
Wireless Technology
RJB uses low energy Bluetooth (Bluetooth Core Specification 4.0) Bluetooth module FCC ID: SH6MDBT42Q
RJB has a Qualified Design ID (QDID) of 125047
Indications for Use
The Ruthless Spine RJB device is intended to measure the angle of surgical instruments in two planes relative to a vertical plumb line in line with gravity. It is indicated for use during lumbosacral pedicle screw implantation in conjunction with applicable spinal instrumentation and as an adjunct to fluoroscopy or intraoperative x-ray. The RJB device is not intended to replace a surgeon’s judgment and has not undergone clinical evaluation. No clinical benefit has been demonstrated or is claimed.
Limitations
No clinical studies, Real World Data (RWD) or Real World Evidence (RWE), or other information are available to evaluate the effect of patient demographics, such as race and ethnicity, on RJB device performance.
Warnings
RJB devices are provided STERILE and must never be reused under any circumstances. Discard any devices that are opened in the operating room but are not used. Re-use of this device may result in serious cross infection or be harmful to the patient.
Do not re-sterilize. Re-sterilization may adversely affect material properties and device performance.
Do not use devices that are received damaged or missing a pull tab.
No modification of the RJB system is allowed.
The RJB device shall only be used by physicians or surgeons trained in lumbosacral pedicle screw procedures. The RJB app may be used by a designated, trained user outside of the sterile field.
During RJB use, the instrument may be tilted in the axial and sagittal planes simultaneously but should not be axially rotated about the slotted handle shaft. Rotation of the RJB device around the handle axis during intraoperative angle measurement can introduce significant error.
The RJB system provides measurements for angles in two planes (axial and sagittal) relative to the vertical gravitational plumb line and does not consider patient anatomy or orientation. The RJB is a spatial tool and not a navigation system providing trajectory guidance.
Contraindications
The device is contraindicated for use in any procedure except for those listed in the Indications for Use.
Cleaning and Sterilization
RJB devices are provided sterile (ethylene oxide sterilization) and are single use. Do not clean or re-sterilize devices. Unused or contaminated devices should be disposed of in accordance with facility protocol. Following use, devices should also be disposed of in accordance with facility protocol.
RJB instruments and handles are provided visually clean and non-sterile and must be sterilized prior to use. None of the provided instruments or handles are reusable. The following sterilization cycle has been validated for the instruments and handles:
Method: Steam
Cycle: Pre-Vacuum
Temperature: 270°F (132°C)
Exposure Time: 4 Minutes
Dry time: 20 Minutes
Instruments should be placed in an FDA-cleared tray and double wrapped in an FDA-cleared sterilization wrap both indicated for the sterilization parameter included in the Instructions for Use. Instruments should be positioned to allow the steam to come into contact with all surfaces. Remove all packaging material prior to sterilization. Only sterile instruments should be used in surgery.
Do not clean or re-sterilize instruments. Unused or contaminated instruments should be disposed of in accordance with facility protocol. Following use, instruments should also be disposed in accordance with facility protocol.
Storage and Handling
Store sterile packaged devices in a manner that provides protection from dust, moisture, insects, vermin, and extremes of temperature and humidity.
Storage Condition: -18 – 50°C, 15 – 90% relative humidity, 70 – 106 kPa
Tablet and App Requirements
The tablet used for running the RJB App is supplied by the user and must be dedicated to RJB App use and not be used for any other purpose. The following are the minimum tablet specifications required for the RJB App:
Minimum Tablet Requirements:
Apple
iPad Pro 11” (4th Generation) and above
Storage: 128GB
iOS: 14 and above
Camera: Camera access is desirable but not required (device barcode may be input manually) Bluetooth: 4.0 and above
Wi-Fi: Is required to download the app but is not necessary for App usage Cellular connectivity: Not required
Android
Storage: 32GB
Android OS: 10 and above
Processor Specifications: 2.0 GHz
Memory: 2GB RAM
Camera: Camera access is desirable but not required (device barcode may be input manually).
If camera function is utilized, minimum camera resolution is an 8MP main camera with auto-focus. Bluetooth: 4.0 and above
Wi-Fi: Is required to download the app but is not necessary for App usage Cellular connectivity: Not required
Tablet and App Installation Instructions
Do not download or install any applications or programs on the tablet apart from the RJB App and security-based tablet software. A Wi-Fi connection is not required for RJB App usage but must be enabled for app installation and updating. Install all tablet operating system updates prior to App use using only known safe and secure networks outside of the hospital. App updates should only be performed when the user is directed to update the app. The user will be informed directly by Ruthless Spine of the need for an update and does not need to rely on Apple or Google Play store update notifications. Do not enable Wi-Fi or connect the tablet to any network during App use. Turn off all other Bluetooth devices in the operating room (use environment) during RJB pairing with the tablet. Do not allow the tablet to be plugged into any external USB computers, networks, or other peripherals during App use. Only factory delivered charging cables and adapters should be used.
1. Download the RJB App from the iTunes store (Apple tablets) or from a one-way download link provided directly to the user (Android tablets) using a known safe and secure Wi-Fi network outside of the hospital.
2. Turn on Do Not Disturb Mode on tablet.
Apple: Go to Settings -> Do Not Disturb and turn it ON. The options should be as follows: Scheduled is OFF, Silence is “Always”, Phone is “Allow Calls From No One”, Repeated Calls is OFF.
Android: Go to Settings -> Notifications -> Do Not Disturb and turn it ON. The options should be as follows: Turn On as Scheduled is OFF, Duration is “Until I Turn it Off”, Hide Notifications -> Hide All is ON, Allow exceptions -> Calls From is “None”, Repeat Callers is OFF, Messages From is “None”, Alarms is OFF, Media Sound is ON, Touch Sounds is ON, Calendar Events is OFF, Reminders is OFF.
3. Turn off Wi-Fi on tablet.
Apple: Go to Settings -> Wi-Fi, Wi-Fi is OFF.
Android: Go to Settings -> Connections, Wi-Fi is OFF.
RJB Operating Instructions
- Turn on the RJB device by removing and discarding the battery pull tab.
- Insert the RJB device into the handle slot. Follow the markings for device orientation. The slot should only allow the device to be inserted in one way.
- Deploy the Ruthless RJB app on a tablet.
- When the app is opened, a tablet screen prompts to pair the RJB device. The RJB identifier can be scanned from the label or manually inputted.
- After pairing the device, a prompt will appear to select the instrument type.
- Once the instrument type is selected, the starting screen will appear. From here, the user can see the angle of the device, hold the angle, offset the angle, edit the instrument name, choose display precision, and watch tutorial videos. Hold and offset features are controlled via touchscreen buttons in the tablet app.
- For use, the instrument should be oriented such that the face of the RJB (marked with ‘UP’ and ‘DOWN’ and visible through the front of the handle slot) is in a plane parallel with the plane of the operating table, without introducing rotation about the instrument shaft. During use, the instrument may be tilted in the axial and sagittal planes simultaneously but should not be rotated about its own shaft. Use the marked face of the RJB to reference the position of the instrument throughout the procedure.
- When using the RJB, it is recommended that the surgeon follow standard fluoroscopy-guided technique, utilizing fluoroscopy as necessary to confirm pedicle screw trajectory intraoperatively. The RJB is a spatial tool and not a navigation system providing trajectory guidance.
- If Bluetooth connection is lost (displayed angles disappear or freeze), restart app. If RJB device does not pair, turn on, or app does not restart, discard the device by disposing in accordance with facility protocol. Revert to standard fluoroscopy-guided technique to complete the procedure.
- To terminate operation of the device, close the RJB app. Remove RJB from the instrument handle and dispose in accordance with facility protocol.
Product Complaints
Any healthcare professional (e.g., customer or user) who has a complaint or who has experienced any dissatisfaction in the product quality, durability, reliability, safety, effectiveness, and/or performance should notify the distributor and/or Ruthless Spine:
Ruthless, LLC dba Ruthless Spine
1438 Arrow Hwy, Ste F Irwindale, CA 91706
855-496-3454
info@ruthlessspine.com
When reporting a complaint, please provide the unique identification number (UDI) or the lot number, your name and phone number, and the nature of the problem.
The surgical technique is available at no charge upon request.
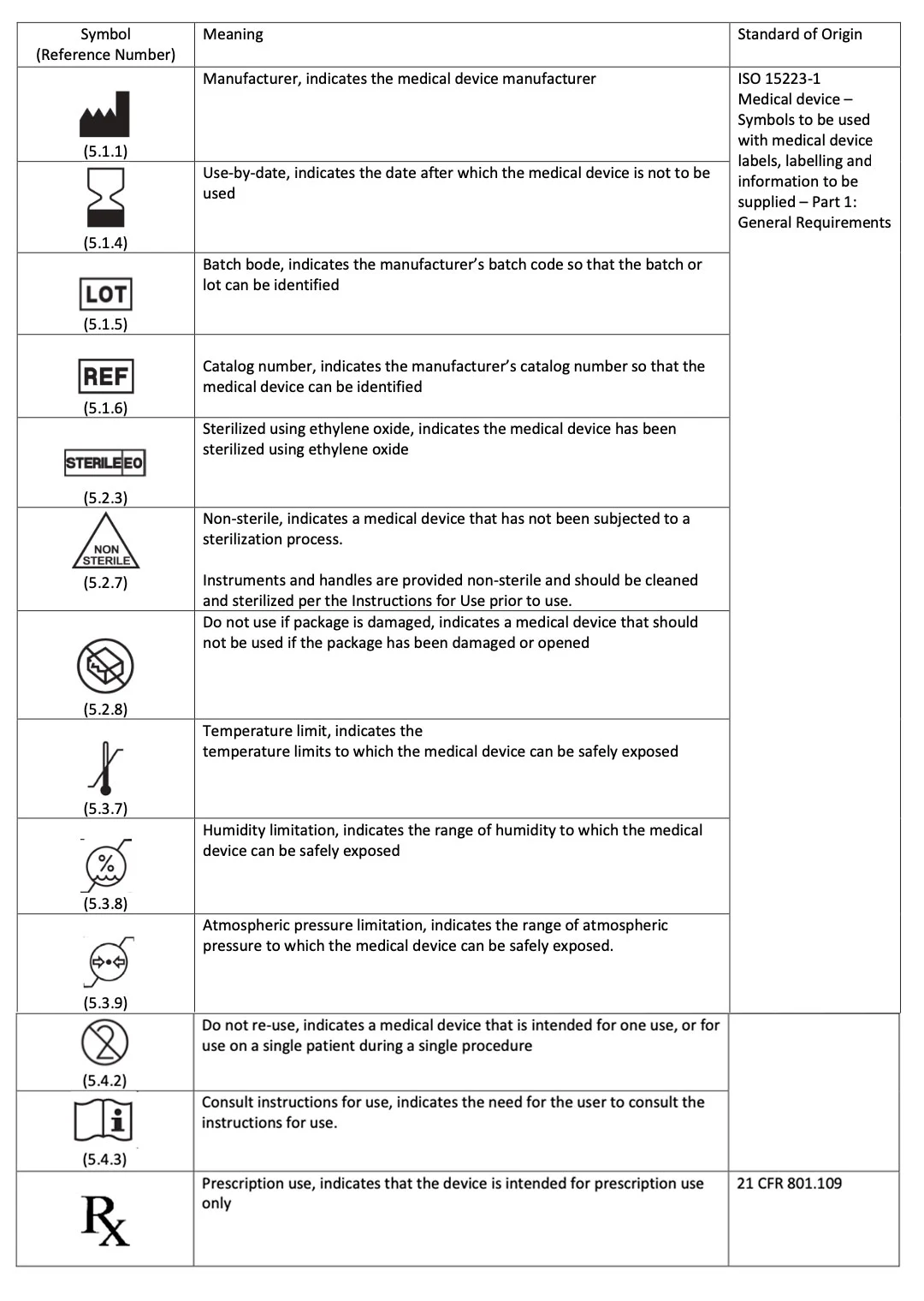
Label Symbols
Appendix A - System Component Identification