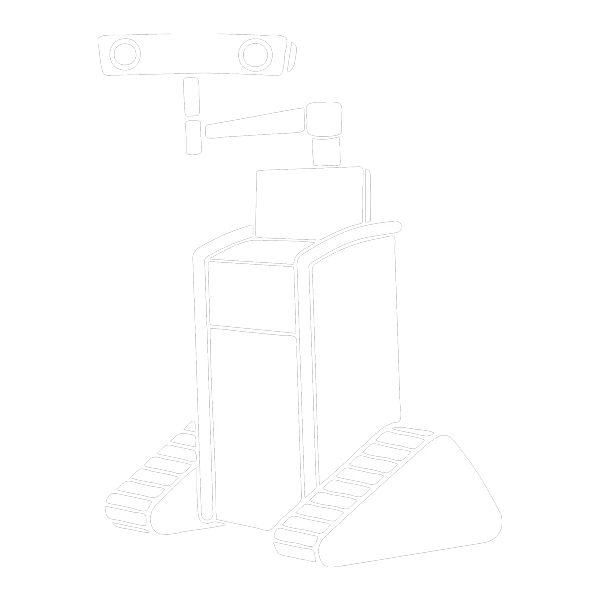
RJB Intraoperative Surgical Angle Measurement Tool: Cleared for Marketing by the FDA
We are thrilled to announce a major milestone in our journey to revolutionize intraoperative surgical angle measurement tools. Ruthless Spine’s RJB device for lumbosacral pedicle screw procedures is officially cleared for marketing by the FDA. This achievement not only validates the effectiveness and safety of our groundbreaking technology but also paves the way for a new era in spine surgery.
About the RJB System
The RJB system, has a simple yet powerful design. It offers spinal surgeons an unparalleled level of accuracy, efficiency, and ease of use. Gone are the days of time-consuming registration and calibration processes. Our device does not require expensive, bulky, and complex machinery. In return, replacing them with a streamlined, off-the-shelf Android or iOS tablet paired with our reliable and intuitive RJB system.

How the RJB Revolutionizes Spine Surgery
One of the key advantages of the RJB device is its ability to not lose connection to the tablet if line of sight is blocked. This feature ensures that surgeons can trust the device to provide continuous and reliable measurements throughout the procedure. The RJB’s Bluetooth-connected module attaches seamlessly to the instrumentation provided with the RJB device. This enables real-time visualization of the axial and sagittal trajectory of the instrument shaft on the tablet screen.
Another notable advantage of the RJB system is its long-lasting battery life. Each single-use RJB module can provide an impressive 8 hours of continuous use. This ensures that surgeons have ample time to perform their surgeries without interruptions or concerns about battery drainage.
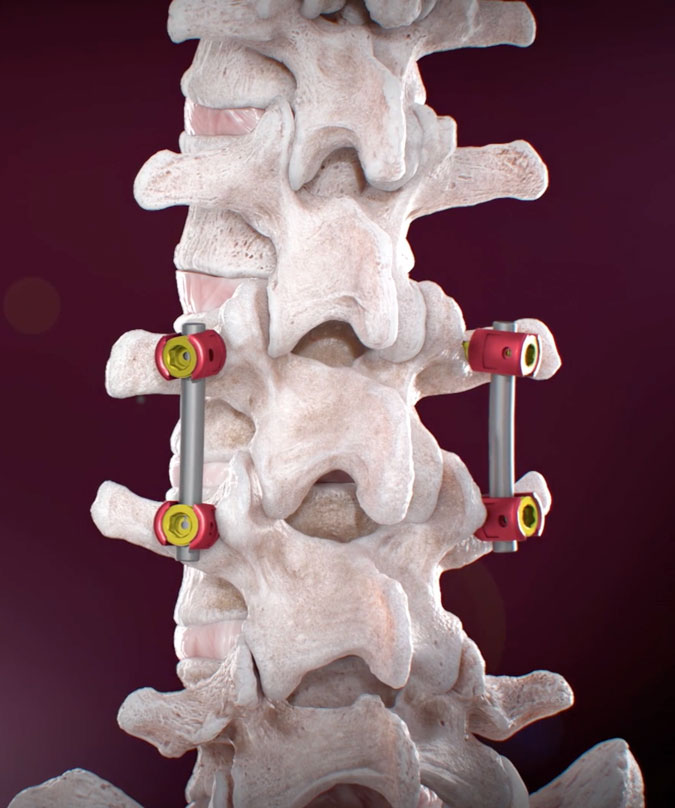
The Future of lumbosacral pedicle screw procedures with the RJB FDA Approved
The FDA approval of the RJB device marks a significant turning point for Ruthless Spine and the field of lumbosacral pedicle screw procedures. This regulatory milestone reinforces our commitment to delivering innovative solutions that simplify and enhance spine surgery. We are excited to provide spine surgeons with a game-changing tool. One that not only improves patient outcomes but also empowers surgeons to unleash their talents and perform at their best.
Looking ahead, we envision a future where the RJB device becomes the gold standard for intraoperative surgical angle measurement tools in lumbosacral pedicle screw procedures. Our ongoing research and development efforts will focus on expanding the capabilities of the RJB system. This involves incorporating advanced technologies to further improve accuracy, efficiency, and patient safety. We aim to continue collaborating with surgeons, medical professionals, and regulatory bodies to push the boundaries of what is possible in the field of spinal surgery.
For the full instructions on using the RJB, refer to our ‘RJB User Guide’.
Conclusion
In conclusion, the FDA approval of Ruthless Spine’s RJB device for spine surgery marks a significant achievement in our mission to revolutionize intraoperative surgical angle measurement tools. With its simplicity, accuracy, and efficiency, our RJB system has the potential to transform the way spine surgeries are performed. We are proud to offer a solution that empowers surgeons and sets a new standard of excellence in the field. As we embark on this new chapter, we remain dedicated to driving innovation and pushing the boundaries of what is possible in spine surgery.